Upcoming Webinars

mRNA Products: An Overview on Strategy for Potency for DS vs DP
Presented by Khaled Yamout, Analytical Sciences, Quality and Manufacturing Consultant, Yamout Chem Consulting, LLC
24th February 2026

Developing a Site-Wide Contamination Control Strategy: From Risk Mapping to Implementation
Presented by Joseph Horvath, PhD, Head of Quality Risk Management at Takeda Pharmaceuticals, Liz Brockson, Aseptic Processing and Sterility Assurance Lead in Global Sterility Assurance and Microbiology at Takeda
02nd March 2026

Assessing the Use of Solid-Phase Cytometry for Rapid Bioburden Testing
Presented by Sophie Drinkwater, Associate Director in Microbiology at AstraZeneca
03rd March 2026
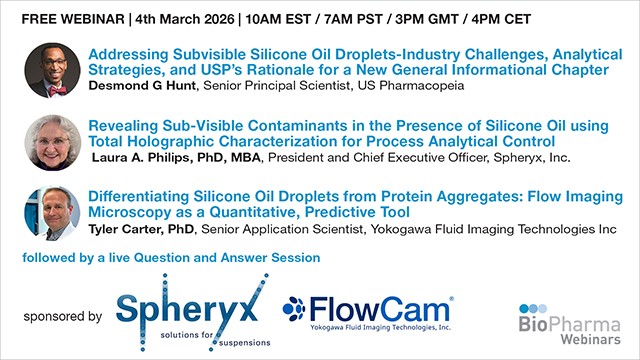
Addressing Subvisible Silicone Oil Droplets-Industry Challenges, Analytical Strategies, and USP’s Rationale for a New General Informational Chapter
Presented by Desmond G Hunt, Senior Principal Scientist at US Pharmacopeia, Laura A. Philips, PhD, MBA President and Chief Executive Officer Spheryx, Inc., Tyler Carter, PhD. Senior Application Scientist, at Yokogawa Fluid Imaging Technologies Inc
04th March 2026
On Demand Webinars

Fatty Acid Extractables/Leachables Profiles as an Indicator of Technical Grade Stearate Acid Additive Origin
Presented by Daniel Norwood, Principal Consultant at Feinberg Norwood & Associates Pharma Consulting
18th February 2026

Enabling Broader Adoption of MAM: Comparison of MAM vs. Conventional Methods
Presented by Diane McCarthy, Senior Director, Science and Standards, Global Biologics Department at US Pharmacopeia, Andrew W. Dawdy, Ph.D. Senior Principal Scientist at Pfizer, Michelle English, Ph.D., Scientific Consultant at Genedata, Ying Qing Yu, PhD, Director of the Biopharmaceutical Applications Team at Waters Corporation
05th February 2026

N-Nitrosamines – Past, Present and Future Through the Eyes of an Analytical Chemist
Presented by Andrew Teasdale, Principal Consultant Nelson Labs
03rd February 2026

Overview and Future Plans of the USP Rapid Microbiological Methods Subcommittee
Presented by Dr David Roesti, PhD. Microbiologist/Facilitator QA/QC at Novartis Pharma AG and Jon Kallay, Senior Technical & Market Development Manager at Charles River Laboratories
29th January 2026

